Accurate Genotyping and Informative Locus Flagging
Accurate Size and Allele Calling
Chimerism analysis using a PCR-based method of short tandem repeat (STR) markers has grown to be the most widely used method for chimerism analysis of stem cell engraftment, over fluorescent in situ hybridization (FISH) and other cytological methods. Human Identity kits for genotyping are relatively inexpensive; STR markers are highly polymorphic and increase the likelihood of finding informative loci for quantitative purposes.
ChimeRMarker® software streamlines chimerism analysis thanks to the following features:
- Accurate size calling and unique pattern recognition technology
- Three easy-to-use dialog boxes to set parameters for consistent analysis of multi-lineage cases
- Time-saving capabilities such as auto-run, linked navigation, quality flagging and audit trail
- Automatically determines informative loci
In addition, ChimerMarker is compatible with all multiplexes, (including but not limited to: Identifiler®, GlobalFiler™ 6-Dye™, PowerPlex®16, PowerPlex®ESI, GenePrint® 24, PowerPlex®Fusion 5 and 6 Dye, Investigator®24Plex)
Setting analysis parameters is accomplished through 3 easy-to-use dialog boxes. Once parameters have been set, they can be saved for future analysis, allowing “single-click” operation of the auto-run feature. Analysis settings include options to correct common chemistry artifacts (Pull-up, Peak Saturation, Spike Removal). ChimeRMarker software detects recipient and donor(s) peaks in post-transplant samples by automatically constructing a case-specific chimertyping panel.
>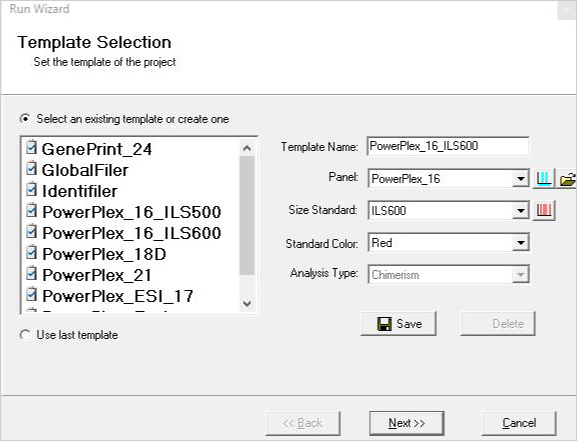
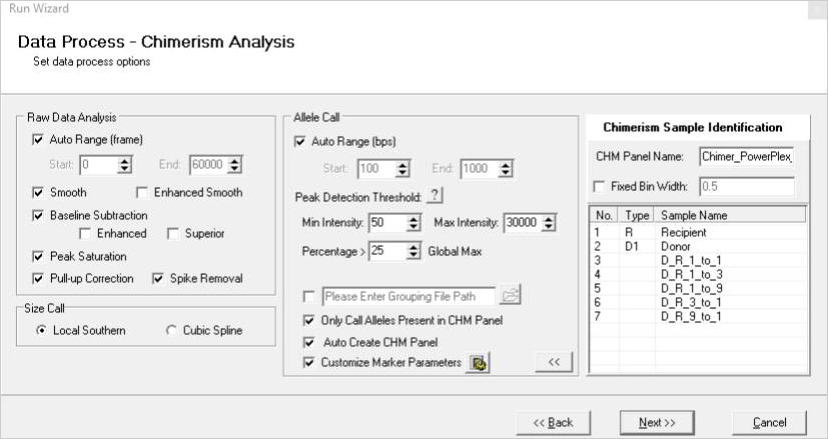
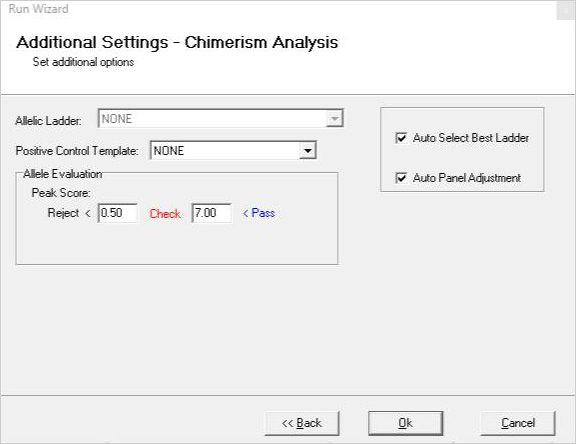
Figure 1: A three-page run wizard provides a user-friendly interface which allows the user to set analysis parameters. Panels for commonly used human identity chemistries and size standards are preloaded in the software.
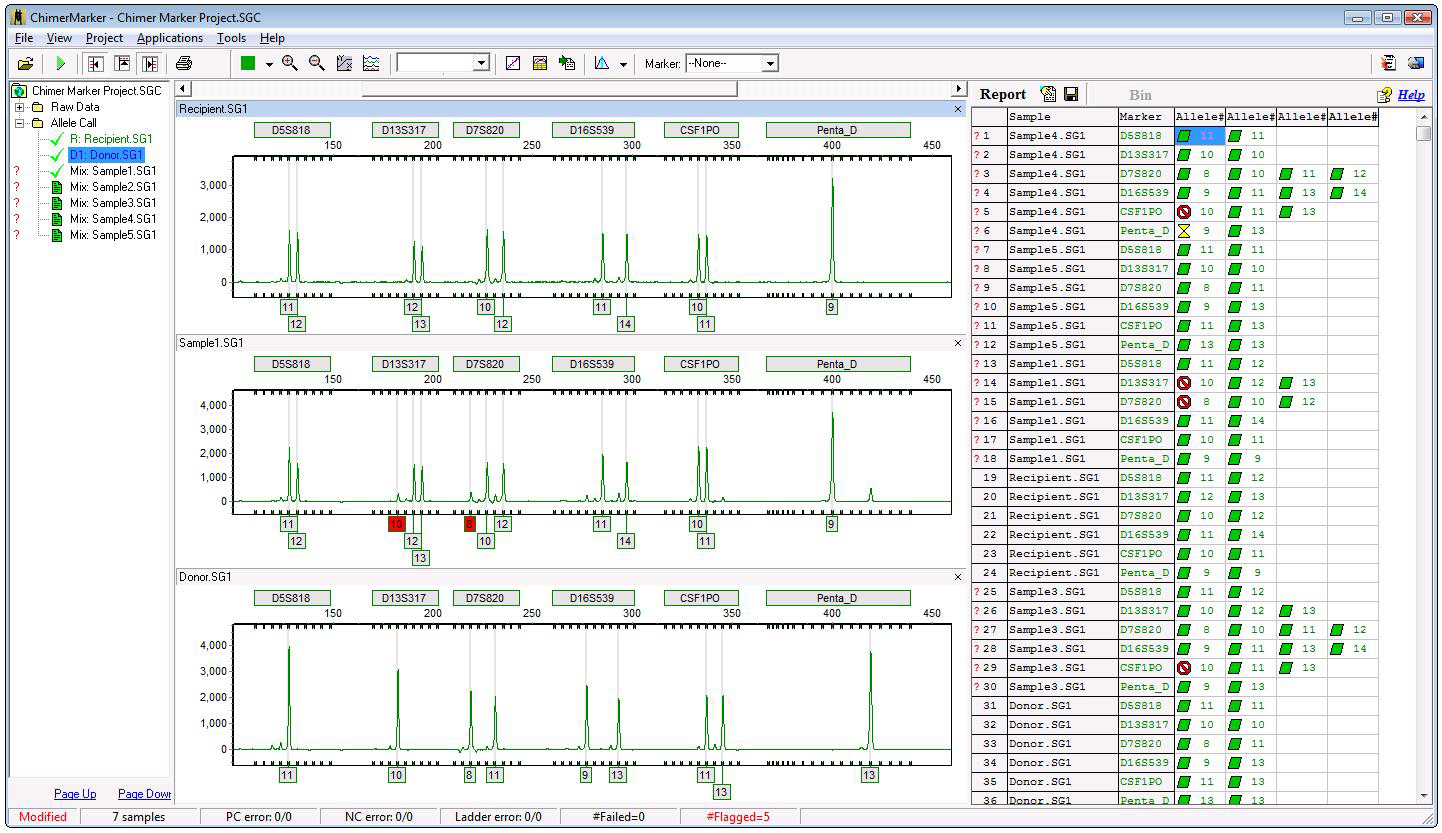
Figure 2: Analysis window displaying genotyping results for three samples: pre-transplantation donor, pretransplantation recipient and two-week post-transplantation sample for recipient T-cells. Two alleles are flagged in red in the PostTX sample—10 for D13S317 and 8 for D7S820—indicating more than three alleles are present in these loci. Allele-calling and artifact-filtering parameters can be configured independently for each locus. Genotyping results (alleles, peak height, peak area, etc.) can be exported as *.txt or *.xls files.
Size Calibration
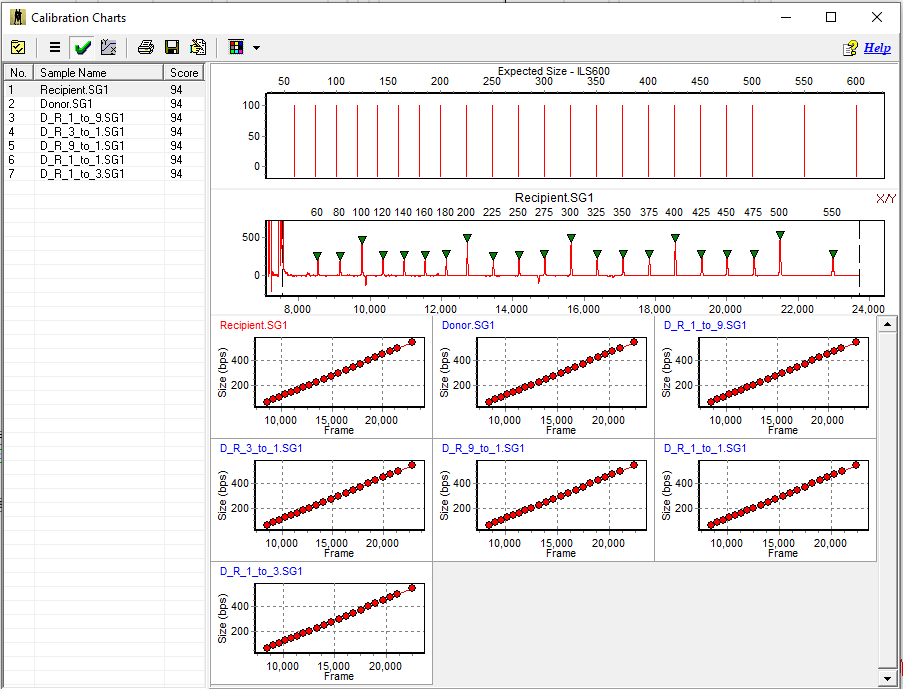
Figure 3: Size call verification is available at a glance in the Size Calibration window. In this example all samples are in the linear range for accurate size calling.
Post-Transplant Sample
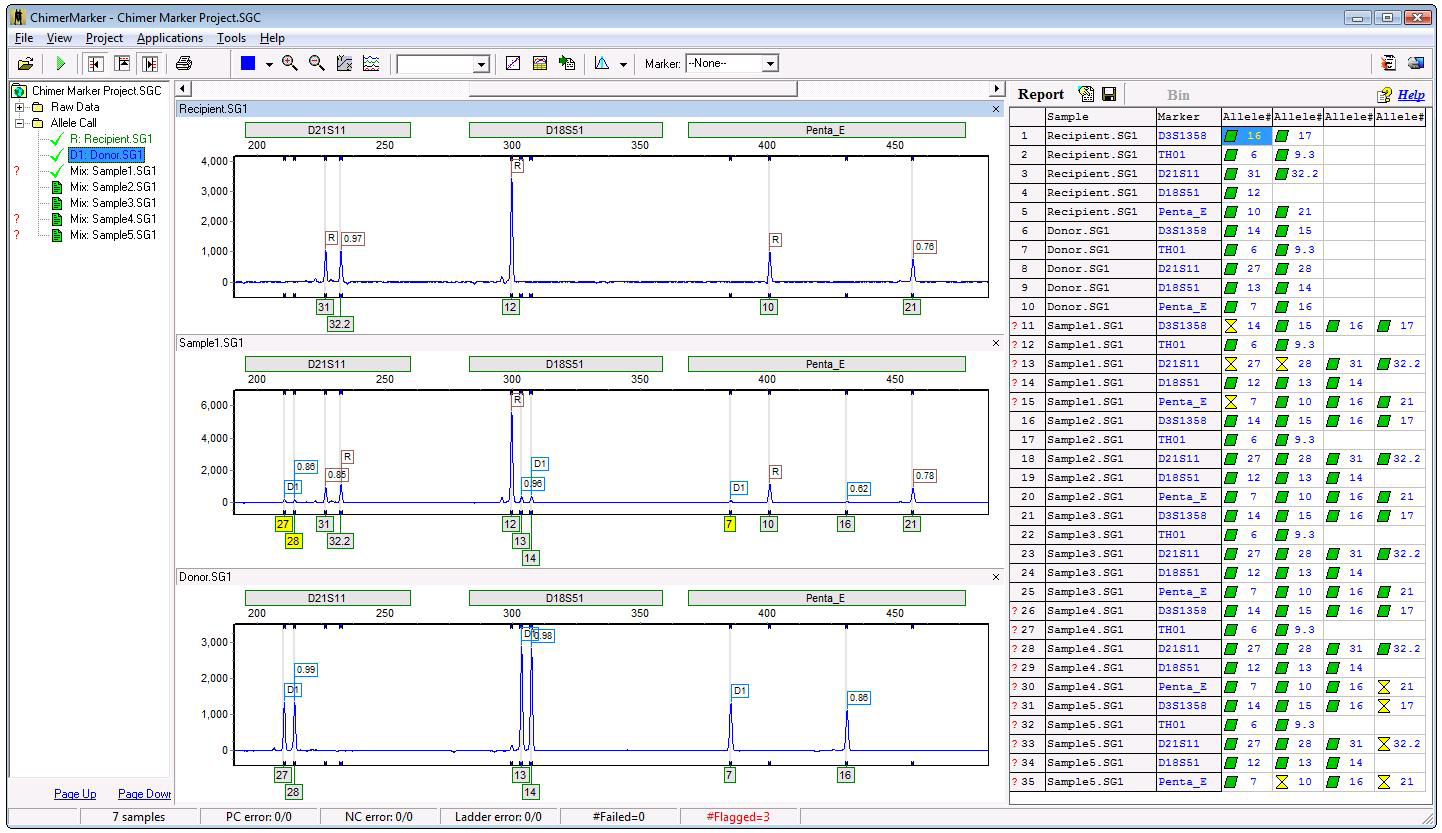
Figure 4: Post-transplant sample analyzed using a chimertyping panel: ChimeRMarker software will differentiate and label peaks for Donor1, Donor2, Recipient, or report the origin of the peaks for shared alleles (D1D2R or D1R, etc.) in each locus. Heterozygous imbalances, by peak height or area, are also calculated for sister alleles of the same locus separately between for each donor and recipient. Chimertyping results [allele calls and origin (donor, recipient or shared), peak area, peak height, etc.] can be exported as *.txt or *.xls files.
Special Multilineage Samples

Figure 5: ChimeRMarker software allows analysis of up to 5 tissue types and multiple donors for each case; provides accuracy by using the same parameters for each sample and saves time by analyzing all samples for a case simultaneously.
Application Notes:













